Introduction
Although the treatment of malignant lymphoma has diversified with the advent of chimeric antigen receptor T-cell therapy and other novel agents, allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains a curative treatment for some patients with relapsed/refractory malignant lymphoma (R/R ML). R/R ML eligible for allo-HSCT often requires urgent transplantation, making human leukocyte antigen (HLA)-haploidentical transplantation with post-transplant cyclophosphamide (PTCY-haplo) or cord blood transplantation (CBT) a promising option when HLA-matched donors are unavailable. PTCY-haplo has recently been reported to achieve similar or better clinical outcomes than CBT for other hematological malignancies, though data regarding R/R ML are insufficient. Therefore, this study compares PTCY-haplo and CBT for patients with R/R ML using the Transplant Registry Unified Management Program 2 (TRUMP2) in Japan.
Methods
This study included adult patients with R/R ML registered with TRUMP2 who underwent PTCY-haplo and CBT for their first allo-HSCT between January 2013 and December 2021. In this study, R/R ML included mature B-, T-, and NK-cell neoplasms, as defined in the World Health Organization's 2017 classification. Patients undergoing allo-HSCT for Hodgkin's lymphoma, aggressive NK-cell leukemia, chronic active Epstein-Barr virus (EBV) infection, systemic EBV-positive T-cell lymphoma of childhood, or adult T-cell leukemia/lymphoma were excluded. The primary endpoint was 3-year overall survival (OS). A Cox proportional hazard regression model for OS was used to estimate hazard ratios (HR) with 95% confidence intervals (CI) in the multivariate analysis. The following factors were included in the multivariate model: donor type, age, sex, performance status, Hematopoietic Cell Transplantation-specific Comorbidity Index score, disease status at allo-HSCT, conditioning regimen, cytomegalovirus seropositivity, and disease linage. The secondary endpoints were 3-year progression-free survival (PFS), 3-year graft versus host disease (GVHD)/relapse-free survival (GRFS), 3-year cumulative incidence of relapse and non-relapse mortality (NRM), 28-day cumulative incidence of neutrophil engraftment, 100-day cumulative incidence of platelet engraftment, 100-day cumulative incidence of grade II-IV and grade III-IV acute GVHD, and 3-year cumulative incidence of any and extensive chronic GVHD.
Results
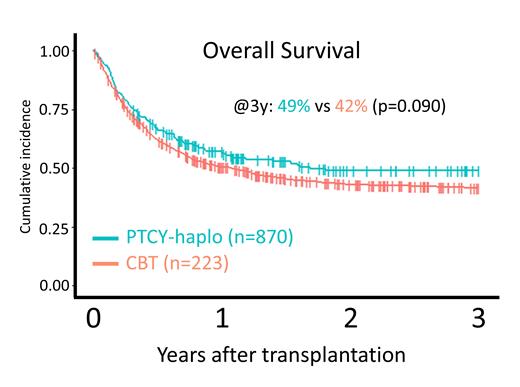
A total of 1093 patients, including 223 who underwent PTCY-haplo and 870 who underwent CBT, were included in the study. The median age was 54 years (interquartile range, 44-61 years), and 616 (56%) patients had B-cell lymphoma. The PTCY-haplo group included older patients, more patients who were positive for cytomegalovirus antibodies in recipients or donors, and more patients who received a reduced-intensity conditioning regimen. The 3-year OS was 49% (95% CI: 41-56%) in the PTCY-haplo group and 42% (95% CI: 38-45%) in the CBT group (P=0.090). The adjusted HR was 0.88 (95% CI: 0.70-1.09, P=0.20). The PTCY-haplo group had comparable 3-year PFS, 3-year GRFS, 3-year relapse rates, and 3-year NRM as the CBT group. The PTCY-haplo group had higher neutrophil engraftment at 28 days (92.8% [95%CI: 88.5-95.6%] vs 81.6% [95%CI: 78.9-84.0%], P<0.001) and platelet engraftment at 100 days (77.6% [95%CI: 71.5-82.5%] vs 71.0% [95%CI: 67.9-73.9%], P<0.001); similar grade II-IV acute GVHD at 100 days and 3-year cumulative incidences of chronic GVHD and extensive chronic GVHD; and lower grade III-IV acute GVHD at 100 days (7.17% [95%CI: 4.27-11.1%] vs 13.6% [95%CI 11.5-16.0], P=0.01).
Conclusion
In this population, PTCY-haplo and CBT result in similar 3-year OS after allo-HSCT. Faster engraftment and less severe acute GVHD were noted in the PTCY-haplo group.
Disclosures
Takayama:Janssen Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria; Celgene: Honoraria; SymBio Pharmaceuticals: Honoraria; Eisai: Honoraria; Asahi Kasei: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria. Kanda:AbbVie: Research Funding, Speakers Bureau; Towa Pharma: Speakers Bureau; CSL Behring: Speakers Bureau; Japan Blood Products Organization: Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Research Funding, Speakers Bureau; AstraZeneca: Speakers Bureau; Human Life CORD: Speakers Bureau; Sumitomo Pharma: Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Takeda Pharmaceutical: Research Funding, Speakers Bureau; Meiji Seika Pharma: Speakers Bureau; Asahi Kasei Pharma: Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding, Speakers Bureau; Saitama Hokeni Kyokai: Speakers Bureau; MSD: Speakers Bureau; Kyowa Kirin: Research Funding, Speakers Bureau; Janssen Pharmaceutical: Speakers Bureau; Sanofi: Speakers Bureau; Pfizer: Speakers Bureau; Chugai Pharmaceutical: Research Funding, Speakers Bureau; Novartis: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Eisai: Research Funding, Speakers Bureau; Precision: Speakers Bureau; FUJIFILM Wako Pure Chemical: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Alexion Pharma: Speakers Bureau; Taiho Pharmaceutical: Research Funding; Shionogi Pharma: Research Funding; Wakunaga Pharmaceutical: Speakers Bureau; Nippon Kayaku: Research Funding; JCR Pharmaceuticals: Research Funding. Atsuta:Meiji Seika Pharma Co, Ltd.: Honoraria; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau; Novartis Pharma KK: Speakers Bureau; Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; JCR Pharmaceuticals Co., Ltd.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal